Many of us Earthlings would like to believe that human life is possible on planets other than our own. One way for this to happen is for humans to try to terraform another planet. That is, to redesign its surface environment so that it is similar to Earth. Our closest neighbour, Venus, is not a good candidate for terraforming because it has a hot, dense atmosphere that would be difficult to remove and because it has lost all but a tiny fraction of its original endowment of water. Mars, the second-nearest planet to Earth, is a somewhat better candidate. Science fiction authors have for many years populated Mars with humans or humanoid races. I grew up reading Edgar Rice Burroughs’s Mars novels and can still remember imagining myself as John Carter, the best swordsman on two worlds, riding thoats and fighting to win the heart of the Martian princess, Dejah Thoris. I’m also still fond of Arnold Schwarzenegger’s movie, Total Recall, at the end of which the Martian landscape is terraformed (in about two minutes) by the release of oxygen from the alien turbidium generators, which Arnold coaxes back to life.
In real life, however, terraforming a planet is not as easy as it made to appear in movies. Turning Mars into another Earth would be extremely challenging for several reasons. We can break these down into three categories: climate, oxygen and other factors.
Let’s start with climate. Mars orbits the Sun at an average distance of 1.52 astronomical units (AU), one AU being the mean Sun–Earth distance. It therefore receives only 43 per cent as much sunlight as does Earth, according to the inverse square law which governs such things. To make the surface of Mars as warm as Earth’s, one would need to either increase the amount of incoming sunlight by, for example, building a set of large, orbiting mirrors, or else beef up the planet’s currently puny greenhouse effect.
The mirror idea is cute and has already been proposed in another science fiction book, Red Mars, by Kim Stanley Robinson. But, if the mirror was also located at 1.52 AU from the Sun, next to Mars, it would need to be bigger than Mars itself to provide the necessary illumination. Constructing such a planet-sized mirror would be a daunting task – one that we are not likely to try within the next few hundred years, if ever.
In real life, terraforming a planet is not as easy as it made to appear in movies. Turning Mars into another Earth would be extremely challenging
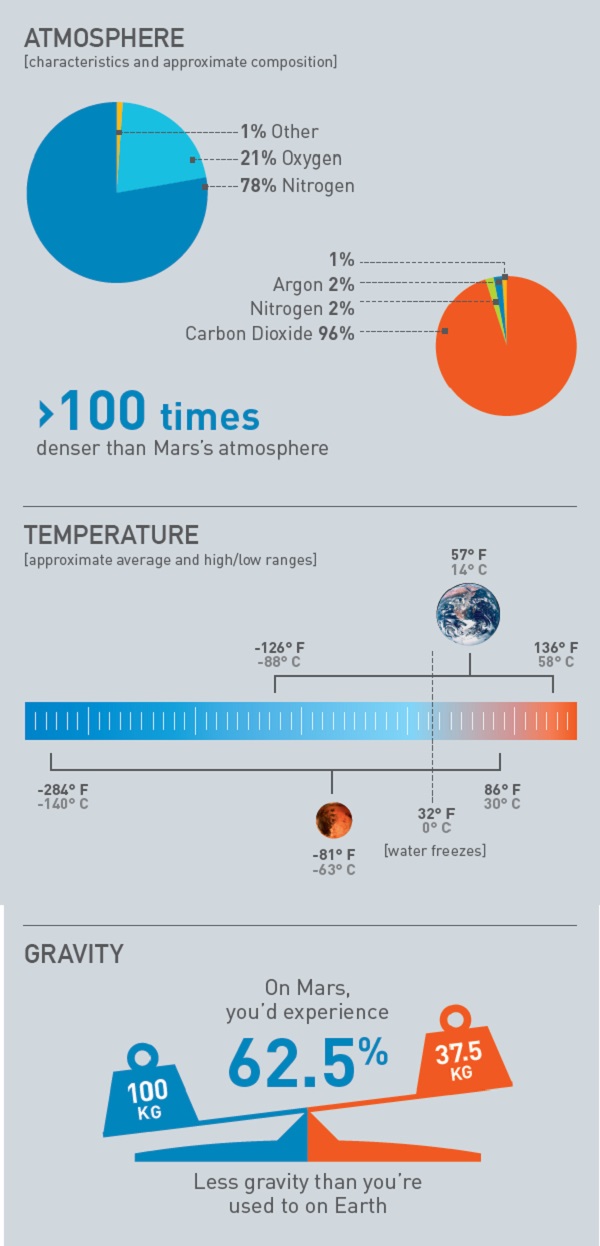
Increasing the greenhouse effect on Mars is also a challenging task. The greenhouse effect involves absorption and re-emission of outgoing infrared radiation by a planet’s atmosphere. On Earth, carbon dioxide (CO2) and water vapour (H2O) are the two main greenhouse gases. Together, they raise Earth’s surface temperature by about 33?C, bringing it up to 15?C. By comparison, the present greenhouse effect created by the thin CO2 atmosphere on Mars is only about 6?C, and the planet’s mean surface temperature is -55?C. So, we would need to provide some 70? of additional greenhouse warming to make the surface of Mars as warm as Earth’s.
Greenhouse gases
Various authors have suggested mechanisms to do this. In their 1985 book, The Greening of Mars, James Lovelock and Michael Allaby suggested that one could warm Mars by importing chlorofluorocarbons (CFCs) from Earth. CFCs are compounds such as freons that have been implicated in the destruction of stratospheric ozone on Earth, but which are also good greenhouse gases. This mechanism, though, would require innumerable and continuing numbers of interplanetary flights because the CFCs break down photolytically into smaller molecules. Manufacturing them on Mars might be somewhat more practical and would likely reduce the cost. But, even if the mechanism did work, one would then have created a chlorine-rich atmosphere that would inhibit the development of an ozone layer, thereby effectively precluding human habitability. This is not a world that one would want to live on.
Other authors have suggested that CO2 would be a better greenhouse gas with which to terraform Mars. Climate calculations show that the red planet’s mean surface temperature could be brought above the freezing point by the addition of about three bar of CO2. (By comparison, Earth’s surface pressure is 1.013 bar, and Mars’s surface pressure is about 0.006 bar.) Getting up to 15?C would require between five and six bar of CO2. Where might this CO2 come from? One idea is to mobilise the CO2 ice stored in the Martian polar caps and in the regolith (the Martian soil). But the amount of CO2 stored in these reservoirs is probably only a few hundred millibars, at best, so even if one released all of it, the surface would remain cold.
Martyn Fogg, a British scientist who has written a book about terraforming Mars, has suggested that there might be much more CO2 stored as carbonate rocks in the deep subsurface. He has also suggested, somewhat tongue-in-cheek, that a good way to release it would be to plant hydrogen bombs at depth all over Mars and then explode them all at once. This would amount to destroying the planet in order to save it. For those of us who are interested in exploring Mars to learn its detailed history and whether or not it has ever supported life, this seems like a bad idea.
 An extreme way to replenish the nitrogen on Mars could be to re-direct nitrogen-rich comets, causing them to collide with Mars. The artist’s impression below shows the close flyby of comet Siding Spring in October 2014, with the NASA orbiters taking cover behind the planet
An extreme way to replenish the nitrogen on Mars could be to re-direct nitrogen-rich comets, causing them to collide with Mars. The artist’s impression below shows the close flyby of comet Siding Spring in October 2014, with the NASA orbiters taking cover behind the planet
An additional problem with the CO2 greenhouse is that CO2 partial pressures above ~30 times Earth’s level shut down the human breathing reflex. Earth has about 0.3 mbar of CO2, so the upper limit on CO2 partial pressure for humans is ~0.01 bar – far too low to provide significant greenhouse warming. Plants can tolerate much higher CO2 levels, and Chris McKay at NASA’s Ames Research Center has suggested that we could, in principle, turn Mars into a plant-habitable world. But would we really want to go to great expense to do this? My guess is that vegetables raised on Mars would be extremely pricey when sold back on Earth. And terraforming Mars just for the benefit of plants seems, again, not worth doing.
What about oxygen (O2)? If one was able to warm the surface of Mars, a lot of ground ice should melt, thereby providing the water needed for growing plants. And plants, of course, produce O2 by photosynthesis. But filling an atmosphere with O2 is still not easy. Most of the O2 produced by photosynthesis on Earth today is consumed by the reverse processes of respiration and decay. (Note that plants, as well as animals, respire. That is how all complex organisms produce the energy required to power their metabolism.)
The O2 present in Earth’s atmosphere is there as a consequence of a small leak of organic carbon into sediments, much of this occurring in shallow ocean water on the continental shelves. Whether or not a warmed-up Mars would have similar places to bury organic carbon is not obvious. Furthermore, the time required to build up Earth-like levels of atmospheric O2, given Earth-like carbon burial rates, is long – about two million years. So, unless we could accelerate this process dramatically, producing the O2 needed for humans to breathe would be a painfully slow process.
Atmospheric issues
How about other factors? There are actually a lot of these to consider. Mars is small and lacks an intrinsic magnetic field, like Earth’s. Hence, it loses atmosphere continually through its interactions with the solar wind. Replacing this magnetic field is once again a daunting task, although one might conceivably be able to block the solar wind by constructing a planet-sized shield at the L1 Lagrange point. (The Lagrange points are points of neutral gravitational stability. L1 is located between the planet and the Sun.)
Mars also lacks plate tectonics and volcanism. On Earth, these processes recycle CO2 back into the atmosphere after it is lost through silicate weathering and carbonate deposition. Without a geologically active planet, we would need to recycle CO2 mechanically, which again would be expensive.
But the most serious problem would be the lack of atmospheric nitrogen (N2) on Mars. N2 constitutes the bulk of Earth’s atmosphere, accounting for 78 per cent of it by volume. Mars used to have a lot of N2, as shown from studies of nitrogen isotopes, but lost most of it to space over time from solar wind stripping and other atmospheric loss processes. And N2, if you think about it, is a very important gas here on Earth. Organisms break it apart into biologically required fixed nitrogen, which is an important component of both proteins and nucleic acids. N2 also serves as a buffer gas that damps the ferocity of wildfires. If an atmosphere is too rich in O2, then flames burn wildly because the convection of air that cools the flame also brings in the O2 that fuels it. Adding N2 to the mix allows convection to carry off heat without generating more energy in the process.
NASA learned this lesson tragically back in 1967 when three astronauts died in a launch-pad fire on Apollo 1. Before that time, NASA used pure O2 in its space capsules because the lower pressure meant that the spacecraft shell could be made weaker and lighter. But they switched to using air immediately after the Apollo 1 fire, having learned the hard way that N2 is a necessary atmospheric component.
 Biosphere 2 is a closed, ecological system in Oracle, Arizona that has been used to explore the viability of biospheres for space colonisation
Biosphere 2 is a closed, ecological system in Oracle, Arizona that has been used to explore the viability of biospheres for space colonisation
Back to Mars again: one might conceivably replenish the N2 on Mars by re-directing nitrogen-rich comets or asteroids and causing them to collide with Mars. But this would take advanced technology that does not currently exist, plus it would likely destroy much of the surface of Mars, just like Martyn Fogg’s hydrogen bombs. I don’t see this as an attractive future for the red planet.
Humans living in cities on Mars might need to spend much of their time underground… this might be a way for scientific expeditions to remain there for long time periods
So, how might humans live on Mars if terraforming is really that difficult? Again, science fiction provides a possible answer. Before the turbidium generators were activated in Total Recall, humans were living there in pressurised, domed cities. There are questions as to whether this lifestyle is actually sustainable, most notably because of the high flux of ionising radiation (galactic and solar cosmic rays) incident on the surface of Mars.
Such radiation is almost entirely blocked on Earth by the combination of Earth’s magnetic field and its relatively thick atmosphere. Humans living in cities on Mars might need to spend much of their time underground to avoid overexposure. This nevertheless seems feasible, and it might be a way for scientific expeditions to remain on Mars for long time periods and to thoroughly explore its surface.
Thus, I’m confident that there will be humans living on Mars in the not-too-distant future. I probably won’t witness this myself, but my grandchildren could conceivably live to see it. In my view, that is the goal we should be shooting for. We should work to establish scientific colonies on the Martian surface and see what we can learn from them.