A technique used on Earth to extract metals from rocks, is now being used on moondust to harvest oxygen; a process that could one day aid future lunar settlers to breathe and to produce rocket fuel.
Moon rocks brought back by astronauts from the Apollo missions confirm that lunar regolith – the dusty surface layer of the Moon that contains soil, broken rock, and other related materials – is made up of 40–45 percent oxygen by weight.
It is the single most abundant element, but despite this rich resource its not easily accessible. Oxygen in moon rocks is locked up chemically as oxides in the form of minerals or glass, so to use it, you need to process it and the easier you can do this, the better.
Returning to the Moon is top of the list for a number of private companies and space agencies who all have different agendas. Some want to just land and conduct scientific experiments, others have ideas of establishing lunar colonies. Either way, as technology improves, longer and longer stays on our nearest celestial neighbour are hotly anticipated.
With this in mind, the European Space Agency (ESA) have set up a prototype oxygen plant at their Materials and Electrical Components Laboratory, housed within their European Space Research and Technology Centre (ESTEC), based in Noordwijk in the Netherlands.
Their aim is to efficiently extract oxygen from moondust and make it useable for would-be moon colonists.
“Being able to acquire oxygen from resources found on the Moon would obviously be hugely useful for future lunar settlers,” says Beth Lomax of the University of Glasgow, who is working on the project as part of her PhD.
Having our own facility allows us to focus on oxygen production, by measuring it with a mass spectrometer as it is extracted from the regolith simulant, Lomax said.
Her work, which is supported through ESA’s Networking and Partnering Initiative, harnessing advanced academic research for space applications, has its roots in molten salt electrolysis.
In chemistry and manufacturing, electrolysis is a technique that uses a direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction.
UK company Metalysis, who specialise in commercial metal and alloy production, have refined this manufacturing process by being able to extract the oxygen while the rock is still in its solid state;
The rock, or in this case moondust, is placed in a metal basket with molten calcium chloride salt to serve as an electrolyte – a chemical compound that is capable of transporting electric charge – and heated to 950°C. At this temperature the rock still remains solid.
A current is then passed through the material, the oxygen is extracted and it migrates across the salt to be collected at an anode (a point of positive charge).
Because the rock doesn’t need to be melted first, like many traditional electrolysis manufacturing processes, the technique developed by Metalysis operates at lower temperatures and lower energy consumption, and it also generates no toxic by-products.
“At Metalysis, oxygen produced by the process is an unwanted by-product and is instead released as carbon dioxide and carbon monoxide, which means the reactors are not designed to withstand oxygen gas itself,” explains Lomax. As part of her PhD, Lomax working at Metalysis to study the process before recreating it at ESTEC.
“We had to redesign the ESTEC version to be able to have the oxygen available to measure. The lab team was very helpful in getting it installed and operating safely.”
At the moment, the extracted oxygen is vented into an exhaust pipe, but will be stored for use after future upgrades to the system are applied.
Another bonus of this process is that the leftover regolith can be converted into usable metal alloys - material that could be used in other applications on the Moon.
“Could they be 3D printed directly, for example, or would they require refining?” says ESA research fellow Alexandre Meurisse. “The precise combination of metals will depend on where on the Moon the regolith is acquired from – there would be significant regional differences.”
This is something that the team are also looking into while they fine-tune the oxygen extraction process.
Ultimately however, the team have long-term goals in mind and a ‘pilot plant’ that could operate sustainably on the Moon is already being reviewed and a first technology demonstration is being targeted for the mid-2020s.
“We’re shifting our engineering approach to a systematic use of lunar resources in-situ,” says Tommaso Ghidini, Head of ESA’s Structures, Mechanisms and Materials Division.
“We are working with our colleagues in the Human and Robotics Exploration Directorate, European industry and academia to provide top class scientific approaches and key enabling technologies like this one, towards a sustained human presence on the Moon and maybe one day Mars.”
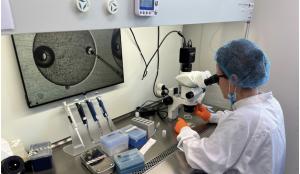
 ESA research fellow Alexandre Meurisse and Beth Lomax of the University of Glasgow producing oxygen and metal out of simulated moondust inside ESA's Materials and Electrical Components Laboratory.Image: ESA
ESA research fellow Alexandre Meurisse and Beth Lomax of the University of Glasgow producing oxygen and metal out of simulated moondust inside ESA's Materials and Electrical Components Laboratory.Image: ESA